Catherine Middlecamp, Department of Chemistry, and Omie Baldwin, University Health Services, University of Wisconsin-Madison, Madison, Wisconsin
A 2004 SENCER Model
Abstract
In 2002, Environmental Chemistry & Ethnicity (3 credits) was created as a new course in the Department of Chemistry, the first in the department to meet the new Ethnic Studies requirement at the University of Wisconsin-Madison. In both 2003 and 2004, the course was taught with a focus on “Uranium and American Indians” by a chemist and a clinical social worker/health professional who is also a member of the Navaho Nation. The course explores the connections between the presence of uranium and the native peoples of the Southwest who lived (and still are living) on the land where the uranium was extracted. Two key issues are addressed: 1.) How policies (public health, occupational safety, environmental protection, and cleanup) are established when national security, corporate interests and the needs of a community come into conflict, and 2.) How indigenous groups in the United States faced and continue to face challenges with respect to both their land and their culture.
The scientific and the cultural, historical, and policy strands of the course are equally weighted and woven together to the extent possible. The science topics covered include the composition of ores and naturally occurring isotopes, mining and milling technology, radioactivity and radioactive decay, radium and radon, ionizing radiation, and nuclear fission and fusion. The cultural and historical issues include the history of the Navaho, their role as workers in radium-based industries, and cancer incidence in the population. The policy questions through which the cultural and scientific factors intersect include the role of the Federal Government in Navaho tribal life, the importance of nuclear weapons and testing in national defense and national security, and environmental and public health problems related to radium. The course is organized around lectures, class discussion, guest speakers and individual projects that students present at the end of the course.
Linking Chemistry and Social Issues
Why is Chemistry & Ethnicity a SENCER model?
What capacious civic questions or problems are addressed in the course?
In 2002, Environmental Chemistry & Ethnicity (3 credits) was created as a new course in the Department of Chemistry. The course title was deliberately left broad enough to allow instructors to select a wide range of topics. In both 2003 and 2004, the course was taught with the topic of “Uranium and American Indians.”
Uranium and American Indians explores the connections between uranium and the peoples of the Southwest who lived (and still are living) on the land where the uranium was extracted. Two key issues are addressed:
- how policies (public health, occupational safety, environmental protection & cleanup) are established when national security, corporate interests and the needs of a community come into conflict, and
- how indigenous groups in the United States faced and continue to face challenges with respect to both their land and their culture.
In one sense, Environmental Chemistry & Ethnicity could be characterized as an “environmental justice” course in that it examines inequities that have arisen from U.S. environmental policies. However, as evident from the description above, the course is more broadly based in that other types of public policy are relevant as well.
This course was the first in the Department to meet the Ethnic Studies Requirement, part of the General Education requirements at UW-Madison. Section 5 of this document, (“What is the Course’s Role in the Undergraduate Curriculum?”) describes this requirement more fully.
What basic science is covered?
This chart lists the scientific topics and the cultural topics. Although listed in separate columns, the topics were woven together to the greatest extent possible. The corresponding issues of public policy (also woven with the topics) are shown as well.
| Scientific Topics | Cultural Topics | Public Policy Issues |
| Uranium Natural occurrence Oxidation states Composition of ores Naturally occurring isotopes |
Four Corners Area The land and the peopleThe Navajo Culture, history, spirituality, and the significance of the land |
Indian Affairs Past and present policies by the U.S. Federal GovernmentDefense National security needs and the Cold War |
| The uranium industry Types of mines Milling processes Tailings & waste |
Navajo uranium minersand the land on which they live | Environmental Protection Site clean-up and remediation |
| Radioactivity Radioactive decay Radioisotopes Half-life Natural abundancesRadium and radon Radioactive decay series Physiological pathwaysIonizing radiation Units Biological effects Dose-response curves Radiation and cancer |
Cancer and its effects on people and their communities The radium dial painters The uranium miners Health care options, especially on the reservation |
Public Health Exposure standards for radioisotopes and how these are setOccupation Health & Safety Identification of an occupational hazard and compensation of the victims |
| Nuclear fission/fusion Nuclear fuel cycle Enriched uranium Depleted uranium Atomic bombs Hydrogen bombs |
Indigenous people Who they are Issues that affect them Examples from around the world |
Defense Policies relating to weapons testing and the Nuclear Test-ban Treaty |
The Course
Syllabus
Course Format
Course prerequisites
This course carries a prerequisite of one of the following general chemistry courses: Chemistry 104, Chemistry 108, Chemistry 109 or Chemistry 115.
Here is a list of topics covered either in the prerequisite courses or in high school chemistry. We assume that you are already familiar with them, but will review them for you during the first three weeks of the course.
- The nucleus, mass number, atomic number, isotopes
- Types of Radioactive Decay
- Naturally occurring radioactive decay series
- Interaction of Radiation with Matter
- Mathematics of Radioactive Decay
The nuclear chemistry topics that we will cover in depth include:
- Uranium: natural occurrence, isotopes
- Uranium ores and the radioactive decay series
- The uranium mining and milling process
- Environmental cleanup and remediation of mines and mills
- Nuclear Waste
- Toxicity of Uranium
- Splitting the Atom: Nuclear Fission
- Exposure to radiation on Earth
- The Nuclear Fuel Cycle
- The Radium Dial Painters
- Radiation as a carcinogen
- Radon and Lung Cancer
Course Design
Semester Overview: Uranium and American Indians
Weeks 1-6
The class opens with a showing of “The Return of Navajo Boy.” This documentary film quickly immerses the viewer in the Navajo language and culture, telling the story of a Navajo boy who was reunited with his family as a man, having years earlier been adopted by white missionaries. The film also portrays the trauma that the boy’s family endured because members worked in the uranium mines.
Following the film, the first four weeks of the course tell two stories. Cathy describes uranium, its properties, and its history. Classroom presentations illustrate where and how uranium is found on the planet, its radioactive properties, its chemical behavior, radon and radium (two notorious decay products), detection and units of radioactivity, half-lives of radioisotopes, the mining and milling of uranium, the nuclear fuel cycle, the waste from the uranium mining process, and, of course, the process of nuclear fission which was one of the reasons uranium was mined, purified and enriched in the first place.
Simultaneously, Omie offers classroom presentations that explore the landscape and peoples of the Four Corners region. Dine: A History of the Navajos begins, “They are the children of Changing Woman. They are called the Navajos. They call themselves Dine.” Omie discusses topics relating to the Dine, focusing on their history and culture, the Indian policies including reservation development (the Dawes Act and the Allotment Act), the policies of the Bureau of Indian Affairs (BIA), the mining development, the Radiation Exposure Compensation Act (RECA), and the social impact of mining, including how it affected their health, their lifestyle and their communities.
Readings during weeks 1-6:
- Memories come to us in the rain and the wind: Oral histories and photographs of Navajo uranium miners and their families (2000). Jamaica Plain, MA: Red Sun Press.
- Eichstaedt, P. H. (1994). If you poison us: Uranium and Native Americans.Santa Fe, NM: Red Crane Books.
- Middlecamp, C., “Nuclear Chemistry,” 2002.
Weeks 7-9
Week 7 marks the beginning of lectures and class discussions on the health effects of radiation. In spring 2004, the guest speakers included:
- Bruce Thomadsen, UW Medical Physics: “The Biological Effects of Ionizing Radiation”
- Doug Brugge, Tufts School of Medicine: “Uranium Mining, the Navajo People and Federal Compensation: Lessons in Fairness”
- Dr. Tien Hoang, UW Medical School: “The Effects of Lung Cancer”
- Dr. Jennie R. Jo, University of Arizona: “Dying While Waiting: The Fate of Navajo Uranium Miners”
- Manuel Pino, Scottsdale Community College: “Uranium Mining at Laguna and Acoma Pueblos”
- Milton Bluehouse, Jr., UW Law School: “The Social, Political, and Legal Context of Navajo Uranium Mining”
Sample readings during weeks 7-9:
- Brugge, D. (2002). The history of uranium mining and the Navajo people. American Journal of Public Health, 92(9).
- Jensen, D. (2000, July). How science ignores the living world: An interview with Vine DeLoria. The Sun, 4-13.
- Uranium mining and processing. Kerr-McGee Nuclear Corporation, Kerr-McGee Center, Oklahoma City, OK 73125.
- Yazzie-Lewis, E. (2001, Fall). Leetso, the powerful yellow monster: A perspective from the plateau. Petroglyph, Canyonland Natural History Association, Moab, UT.
- Hively, W. (2002, December). Is radiation good for you? Discover, 75-80.
- Selected articles, Voices from the Earth, a quarterly publication of the Southwest Research and Information Center, Albuquerque, NM.
Weeks 10-15
During the last 6 weeks of the semester, students make presentations on topics that weave together chemistry and indigenous culture. In each class period (75 minutes), two students share the time, presenting their work, answering questions, and leading the discussion. Students also provide a list of questions to guide study and discussion of the issues. Each presentation is accompanied by a 4-5 page paper (not including citations and figures).
The topic for the first of these presentations will be “indigenous people,” answering questions such as: What is an indigenous person? Who are the indigenous people in the U.S.? What issues face indigenous people, especially in the U.S., but as time permits, with a world-wide perspective. We ask for two volunteers to split this first topic in any manner they wish.
Students choose the topics for the remainder of the presentations. Each one must explore in more depth the issues raised in If You Poison Us and The Dine, and/or raise new issues.
Examples of topics selected by students in the previous semester include:
- Yucca Mountain: Storage of nuclear waste on or near tribal lands
- Depleted Uranium (DU) and where the U.S. military tests/deploys it
- The Radiation Exposure Compensation Act and the Navajo People
- The after-effects of nuclear weapons testing on the Marshall Islands
- Weapons testing and tribal lands in the U.S.
- Spirituality and the land
- The Kerr McGee Corporation and the uranium miners
Pedagogical Methodologies
Understanding Context
This course, “Uranium and American Indians,” teaches through a real-world issues that affects a community of people to the underlying scientific concepts. In taking this approach, our agenda was to give science and race/ethnicity an equal intellectual footing.
One way to represent our approach to teaching “Uranium and American Indians” is as follows:
The further out one moves from the core, the richer the historical, social, ethnic, economic, and/or political context of the material.
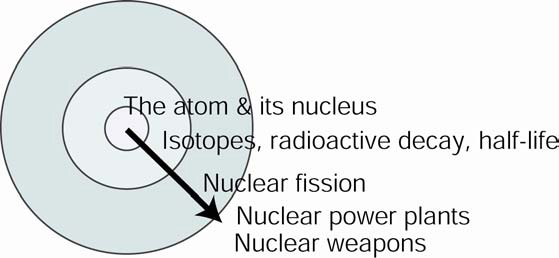
To better understand how our course (and the diagram that represents it) differs from the norm, we show another representation that is more typical of a general chemistry course:
Such a course teaches “outwards,” beginning with core chemical facts, concepts, and rules. Once these have been established, the instructor then may include real-world applications such as nuclear power plants or nuclear weapons. Other applications, of course, are possible as well. For example, a course that prepares health professionals many include applications in nuclear medicine such as PET or radiation therapy.
As evidenced by most text books, general chemistry courses teach “outward” only to a limited extent. The reasons for including fewer “real-world” examples (i.e., less context) vary, but presumably relate to instructors wanting to “cover” more topics, trading depth for breadth. Another reason may be that instructors prefer to stay within the comfort zone of their scientific discipline and not venture into the wider societal (and less tidy) issues.
One notable exception in general chemistry is the textbook for nonscience majors, Chemistry in Context, a project of the American Chemical Society. Now going into its 5th edition, this text teaches chemistry through the real-world issues that face our society. Keeping with the example of nuclear chemistry, the nuclear chapter of Chemistry in Context opens with a discussion of the uranium pellets that fuel a commercial nuclear power reactor. The approach could be represented as follows:
Since its inception, Chemistry in Context has departed from the norm in two significant ways. First, it teaches inwards from an issue, rather than outwards from core principles. And second, it includes more layers of context than a typical general chemistry course. In fact, in 1999 Chemistry in Context was selected as one of the earliest of models for the SENCER project.
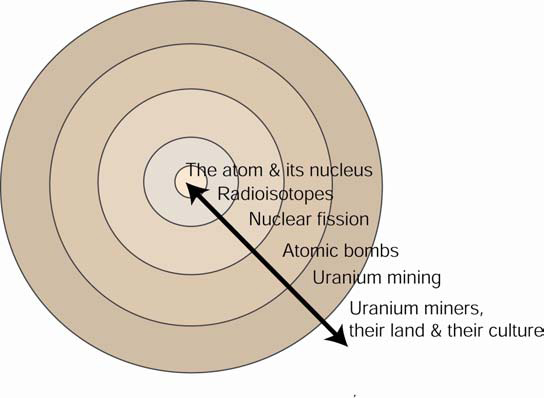
Uranium & American Indians utilizes the approach of Chemistry in Context, but departs from it in two ways. First, the course moves outward to the issues of nuclear weapons rather than those of nuclear power. Second, and more significantly, the course continues “outwards” until it reaches a community of people who were affected by the construction of nuclear weapons.
The issues raised in the book If You Poison Us led us outward to this higher degree of context. For example, where does the ore for one of the possible fissionable fuels (uranium-235) for nuclear weapons come from? Back during the Cold War, who mined it, milled it, processed it and where did this take place? And what happened (and is happening today) to these people and to the land on which they lived and worked? What public policies protected workers, their land and their communities? By asking questions such as these, the Navajo people who mined and milled the ore become intimately joined to chemical content.
Thus, by responding to the questions posed by a community of people and in turn moving outward from the discipline, a cultural and societal context becomes established. In so doing, ethnic communities claim a rightful place in the science curriculum. To fully understand the impact of nuclear fission, nuclear weapons, uranium mining and nuclear waste, the science behind these requires an understanding of the people involved and their culture. Likewise, for the people to undersatnd what happened to them and to their land, an understanding of the scientific principles is required.
This model, with science at the center, well represents the teaching of science through a capacious and complex issue. However, we realize its limitations. First, it is too simplistic, in that the teaching trajectory is far from linear. As you will see in the syllabus, we do start at the “outside” as with a film about the uranium miner, their land, and their deaths from lung cancer. To understand these deaths, we reach both inward towards scientific principles. But as the course progresses, the discussions move easily in both directions along the arrow. Thus, it might be better represented as:
Second, we realize that the model is chemistry-centered, which is not true to the actual course dynamics where the course content centered both on chemistry and Navajo culture. To address this, we might use this alternate representation:
Here, the issues of public policy are the point of overlap between the issues facing a cultural group, and the scientific principles related to these issues. This same information is presented in more detail in the chart in the previous section.
Evaluating Learning
Each week, students were given study questions to help them prepare for the biweekly quizzes. Here are examples. The complete set of study questions can be found on the calendar at the course web site.
What kind of Student and Course Assessment Strategies Are Used?
Assessment of student prior knowledge
Both years, we assessed student background knowledge of nuclear chemistry and Navajo history by using an anonymous survey administered on the first day of class. In general, the students performed better on those questions relating to science than they did on ones relating to culture. In fact, the students’ performance on the questions showed that they knew almost nothing about Navajo history and culture. Fewer than half could correctly label the four states that comprise the Four Corners region, and very few could list an event in Navajo history. Given this, we dedicated class time to familiarizing the students with the geographic region, including features such as the state boundaries, major highways, cities, vegetation, and land formations.
Results from selected questions are summarized below, combining the data from the spring semesters of 2003 and 2004.
| Questions | Number of correct answers | Number of incorrect answers |
| What does the 238 in Uranium-238 mean? | 21 | 24 |
| Define the term “half-life.” | 35 | 10 |
| U-238 is an alpha emitter. Write the nuclear reaction. | 14 | 31 |
| What is meant by the term “ionizing radiation?” | 16 | 29 |
| What is the relationship between these two words? Navajo and Dine (Dineh). | 9 | 36 |
| Draw and label the four states of the Four Corners Region on the figure below. | 16 | 29 |
| Describe any event in the history of the Navajo people. Give as much detail as you can. | 3 | 42 |
Presentation
Writing
Exams & Quizzes
Class Presentation Peer Assessment Form
Paper Final Draft Assessment
Concluding Thoughts and Challenges
From the outset, this course challenged the instructors, both intellectually and personally. The challenge was intellectual in the sense that the course blended equal parts of culture and chemistry. Such a course had never been taught before at our institution, and only because of a campus wide general education requirement were we able to find a home for it in the curriculum. Both the Department of Chemistry and the Ethnic Studies Review committee scrutinized the course, as a science course that met the ethnic studies requirement was unfamiliar (if not implausible) to both.
The content challenged the instructors as well. They wanted to give equal weight to chemistry and culture, yet never isolate one from the other. Difficulties in structuring the course content and process were compounded by the fact that Omie Baldwin knew little or no chemistry, and Cathy Middlecamp knew little or no Navajo history or culture. Throughout both the design and the teaching of the course, the instructors were truly dependent on each other in that the expertise of both of them was required.
It was possible to meet these intellectual challenges, as this document demonstrates. We extend the same challenge to others: to blend culture and science, thereby enriching students’ (and our own) understanding of the complexities that connect them. A chemist quipped to Cathy, “But I don’t have a Navajo colleague to teach with me,” perhaps trying to get off the hook for ever teaching this type of course.
But success at this task is not dependent on having any one particular colleague. Rather, the key lies in joining a person who knows science with a person who knows a particular cultural issue. Many of the issues that face communities of people could serve as a basis for course. Consider, for example, coal miners in Appalachia, AIDS patients in the southern United States, or indigenous people near the oil fields in Alaska.
In thinking about the possibilities, this representation may serve as a useful as a starting point.
The men and women affected by coal mining could be placed at the outer ring, and you could teach inwards to air quality, fuel combustion, chemical reactions and stoichiometry. Along the path you may encounter the black lung disease of the miners, the acid deposition from burning coal, and perhaps societal needs for energy. Again, the possibilities are limitless.
However, to be honest, we must admit that some of the personal challenges we encountered did not leave us with the same sense of satisfaction. The greatest difficulty lay in the fact that teaching the course drained the personal resources of both instructors. By and large, this situation arose because too many duties were added to the instructors without subtracting others. Omie Baldwin received 25% release time, yet as a health professional new to the world of teaching/learning, this was insufficient to compensate for the time and energy the course (and its students) required of her. Cathy Middlecamp contributed her time as an overload, with no reduction of previous duties. Even with a 25% graduate Teaching Assistant to assist her, the combined workload was not manageable.
Again we offer the challenge to the wider community. If we are to create new knowledge or to create new ways of configuring “old” knowledge, we need to find workable professional pathways for this type of scholarship. And for the sake of our students, our professions, and our planet, we need to connect this science with the issues that face us and our world. We have struggled with the issues. And we look forward to join with all who wish to be part of this conversation in the years to come.
Background and Context
Contacts
Cathy Middlecamp
Department of Chemistry
1101 University Avenue
Madison, WI 53706
chmiddle@wisc.edu
Omie Baldwin
University Health Services
905 University Avenue
Madison, WI 53715
obaldwi1@facstaff.wisc.edu
Institution
University of Wisconsin-Madison
Teaching goals and philosophy
By Cathy Middlecamp
(excerpts from “The Art of Engagement,” CONFChem, Fall 2003)
Introduction
What engages students? And what engages us in engaging them? Both of these questions need our attention as we consider how and why we should teach chemistry to non-science majors. The stakes are high. If students do not engage, they are unlikely to learn. And if we do not engage, we are unlikely to engage our students. Furthermore if we do not engage, we miss out on opportunities to learn ourselves. Thus, the engagement of all involved in the teaching and learning processes would seem to be a worthy goal.
Worthy or not, engagement is no simple process. It involves a commitment of self and energy. And even with such a commitment, engagement may remain an elusive goal. These observations serve as the rationale for the assertion made in the title, namely, that engagement is an art. Engaging one’s students is not simply a matter of following a set of rules and/or persisting. Rather, like any art, engagement requires creativity and must be developed and practiced over time.
The Courage to Engage
Frankly, I never expected to be so engaged in teaching non-science majors. In planning a career path, this possibility never crossed my mind. But once my students engaged me, I was hooked and in this, there is a certain justice. In my earlier attempts at finding a chemical “hook” that would engage my students, I found myself on the hook as well.
Parker Palmer, in his book The Courage to Teach, writes: “We did not merely find a subject to teach – the subject also found us” (p. 25). I agree. Real-world topics such as nuclear radiation, plastics and recycling, and smog found me. With these and other issues that deeply affect all of us on this planet, I became engaged. In turn, I want to engage my students in their complexities. As a friendly corollary to Parker Palmer’s statement, I also would want to assert that we do not merely find students to teach, the students also find us. And once they do, a certain amount of courage is required on our part.
Why courage? For at least three reasons. First, to engage students you have to know and connect with your students. This is not for the faint of heart, in the sense that you will be drawn into their worlds in ways that are perhaps unfamiliar or uncomfortable. Second, once you engage students, they will engage you as well. This has a cost in time and energy, and you will discover which boundaries you can cross, which personal frontiers you can negotiate, and which, for a variety of reasons, you simply cannot. Thirdly, and to me perhaps the most exciting realization, is that engagement with what you teach carries an intellectual challenge. Let me explain by offering an analogy. In teaching a first-year course to non-majors, I feel much more like I am conducting one at the graduate level. My course carries the same challenge as a graduate course in keeping current. For example, with a topic like ozone depletion, I add or subtract from my notes each time NASA makes announcement about this year’s ozone hole, whenever nations meet to further amend the Montreal Protocol, when Freon smuggling reaches a new high, when an industrial refrigerant accident tragically kills works by ammonia inhalation, and/or when new chemical information about the behavior chlorine in the upper atmosphere is released. Whew! I sometimes long for the days when I could simply pull out the same old buffer titration problems off the shelf year after year. It takes courage to commit to a course that will in turn commit you to a serious degree of study.
Practicing the art of engagement
I return to an assertion made in the title: Engagement is an art worthy of a lifetime of reflection and study. As such, it comes neither easily nor cheaply, but rather with a personal commitment and a willingness to practice. This art involves making good choices in regards to the content that is taught (and not taught), in regards to one’s personal involvement in the teaching process, and in recognizing the subtleties and challenges of the larger learning context for both our students and for ourselves.
Think for a minute about practicing any art: a musical art, a martial art, or a medical art. The “practice” needed for any of these involves a significant commitment of self. Equally importantly, these arts require a willingness to enter into the practice as a beginner. Acknowledging one’s status as a beginner can offer tremendous freedom. Beginners are free to be just that, allowing themselves to at times act clumsily or ineptly. Beginners also are free to seek out experts. Fortunate is the beginner willing to practice in the company of one who shows mastery of the art!
To the extent that we expect to quickly and easily engage our students, we deny the very art form of engagement. We also lose the freedoms that can and must be granted to beginners. Admittedly some are born with incredible amounts of raw talent, perhaps “born” as painters, gymnasts or composers. Personally, I believe that the art of engagement can be won over time and through practice. With one action, we may overengage at too great a personal cost. With another, we under-engage or inappropriately engage, thus disconnecting from our students. Through practice, we find a path of engagement that works both for us, for our students and for the world that connects us.
Where is the course taught?
The University of Wisconsin-Madison is a public land grant institution. It is the largest campus in the University of Wisconsin System that includes 13 universities (“comprehensives”) and 13 two year schools, and an extension.
The UW-Madison web page provides a link to these quick facts:
- Location: Madison, Wisconsin
- Founded: 1848 (First class: February 1849)
- Campus: 933 acres (main campus)
- Enrollment: 41,588
- Budget: $1,696,085,152
The student profile (Fall 2003) is listed as:
| Men | 19,876 | 47.8% |
| Women | 21,712 | 52.2% |
| Ethnic minority students | 4,108 | 9.9% |
| African American | 994 | 2.3% |
| Asian American | 1,838 | 4.4% |
| Native American | 230 | 0.6% |
| Hispanic | 1,046 | 2.5% |
| From Wisconsin | 25,974 | 62.5% |
| From other U.S. States | 12,077 | 29.0% |
| International students | 3,571 | 8.6% |
| U.S. states represented | 50 | |
| Countries represented | 120 |
| Average composite ACT (freshman class) | 27.5 |
| Average SAT | 1,260 |
| Average high school class percentile rank | 88.7 |
At the UW-Madison, approximately 2500 students take first year (“general”) chemistry each fall.
What is the course’s role in the undergraduate curriculum?
This course fulfils a general education requirement for ethnic diversity in the U.S. However, not all students take it for this reason. As you can see by the student comments in the assessment section, some non-science majors took it for other reasons (such as a means to continue their study of how science relates to societal issues).
How does the Course Advance or Engage Institution-Wide Initiatives?
In part, this question is answered in the previous section. This course contributes to the UW general education program in that it meets the Ethnic Studies Requirement. This requirement was instituted back in the 1980s when faculty and administrators nationwide were responding to the issues of race/ethnicity that were facing them and their students.
More recently, our institution has committed to Plan 2008, “a broad and aggressive plan for what we need to do to make institutional improvements necessary to achieve greater diversity on campus.” One of the goals of Plan 2008 is to:
“Foster institutional environments and course development that enhance learning and a respect for racial and ethnic diversity.”
Our course is in service of this goal. As we acknowledge in the last section of this document, funding for Omie Baldwin’s release time was granted through Plan 2008.
The full text of Plan 2008 is available at: http://www.provost.wisc.edu/documents/plan2008.pdf
Related Resources
E-Newsletters:
SENCER Innovations Featured At ACS Meeting by Trace Jordan – September 2003, p. 6
First Results from the SENCER SALG Reported – March 2004
Environmental Chemistry and Ethnicity: Uraniaum and American Indians – Catherine Middlecamp and Omie Baldwin – November 2004
Seventeen Midwestern Schools Practice the “5 R’s”-Reuniting, Reporting, Recruiting, Recommending, and Regionalizing- at SENCER Symposium in Chicago – February 2005 p. 6-8
Learning Chemistry Through Policy Issues and Civic Engagement:
A Report from the American Chemical Society Meeting in San Diego April 2005, p. 7-8
Green Chemistry: Not an Oxymoron – April 2005, p. 9-10
SSI 2005-Preview of SENCER Alumni Symposia: A Celebration of Work – July 2005, p. 7
How Can Global Experience Improve Science Education? Ask The Experts Among Us – August 2005 p. 4-5
Backgrounders:
“Implications of Learning Research”
“Reinventing Myself as a Professor”
Textbooks:
Eichstaedt, P.H. 1994. If you poison us: Uranium and Native Americans. Photographs by Murrae Haynes. Santa Fe, NM: Red Crane Books.
Iverson, P. (2002). Dine: A history of the Navajos. Albuquerque, NM: University of New Mexico Press.
Middlecamp, C. 2002. “Nuclear Chemistry.” Unpublished.
Memories come to us in the rain and the wind: Oral histories and photographs of Navajo uranium miners and their families. (2000). Jamaica Plain, MA: Red Sun Press.
Resources:
“The return of Navajo Boy.” Documentary film. Information available at navajoboy.com
Brugge, D. (2002). The history of uranium mining and the Navajo people. American Journal of Public Health, 92(9).
Jensen, D. (2000, July). How science ignores the living world: An interview with Vine DeLoria. The Sun, 4-13.
Kerr-McGee Nuclear Corporation. Uranium mining and processing. Oklahoma City, OK.
Yazzie-Lewis, E. (2001, Fall). Leetso, the powerful yellow monster: A perspective from the plateau. Petroglyph (Canyonland Natural History Association, Moab, UT).
Hively, W. (2002, December). Is radiation good for you? Discover, 75-80.
Selected articles, Voices from the Earth, a quarterly publication of the Southwest Research and Information Center, Albuquerque, NM.
Articles about “Chemistry & Ethnicity”
Carlson, E. (2002, December 10). Course blends chemistry, American Indian studies. Wisconsin Week. Available at http://www.news.wisc.edu/8111
Osgood, A. (2003, March 31). Chemistry course brings together science, ethnic studies. The Daily Cardinal.